The LYMPH-Trial
Surgical treatment compared to “non-surgical” treatment of chronic lymphedema after breast cancer therapy
Why are we conducting the LYMPH-Trial?
We are looking for better treatment options for lymphedema after breast cancer therapy.
To date, lymphedema has been treated purely through conservative methods, such as lymphatic drainage, physical exercises, and compression garments. However, this approach only alleviates the symptoms and cannot achieve a lasting improvement. It is costly and must be sustained throughout the rest of the patient's life.
There are microsurgical operations as a treatment for lymphedema.
Two commonly used surgical techniques around the world, called “Lymphovenous Anastomosis (LVA)” and “Vascularised Lymph Node Transplantation (VLNT)” are used to improve the drainage of lymphatic fluid. These procedures can be combined with surgery to remove body fat (liposuction). Several national and international trials have shown that both techniques are effective. That is, patients undergoing these surgeries reported a decrease of lymphedema in the arm and an improvement in their quality of life. You will find more information about the treatments on this website under “Treating lymphedema”.
We need scientific evidence to ascertain the better treatment for lymphedema.
Relying solely on individual reports is insufficient. We need clear scientific evidence demonstrating that microsurgical treatment in combination with conservative therapy genuinely improves the quality of life and reduces lymphedema in patients with chronic lymphedema following breast cancer treatment.
What does the trial aim to achieve?
1
Therapy
We need strong evidence, enabling doctors and health insurance companies to make well-informed decisions about recommending surgery for patients.
2
Quality of life
We want patients who suffer from chronic lymphedema after breast cancer treatment to lead a better life in the long term.
3
Information
We will share the outcomes of our research openly, allowing patients worldwide to access and benefit from the study results.
Who can
participate?
Participants of all genders are welcome in the study if they meet the following criteria:
You are over 18 years old.
You have been diagnosed with breast cancer.
A doctor has confirmed your diagnosis of chronic breast cancer-related lymphedema.
You have been undergoing treatment for chronic breast cancer-related lymphedema for a minimum of 3 months. This treatment involves conservative therapy such as lymphatic drainage and compression garments.
You have not undergone surgery for the treatment of chronic breast cancer-related lymphedema.
How do we proceed?
If you choose to participate, you will be randomly assigned to one of two groups:
Group A will undergo surgery and receive conservative therapy.
Group B will continue with conservative therapy alone.
At the end of the intervention, group B can have surgery if they wish.
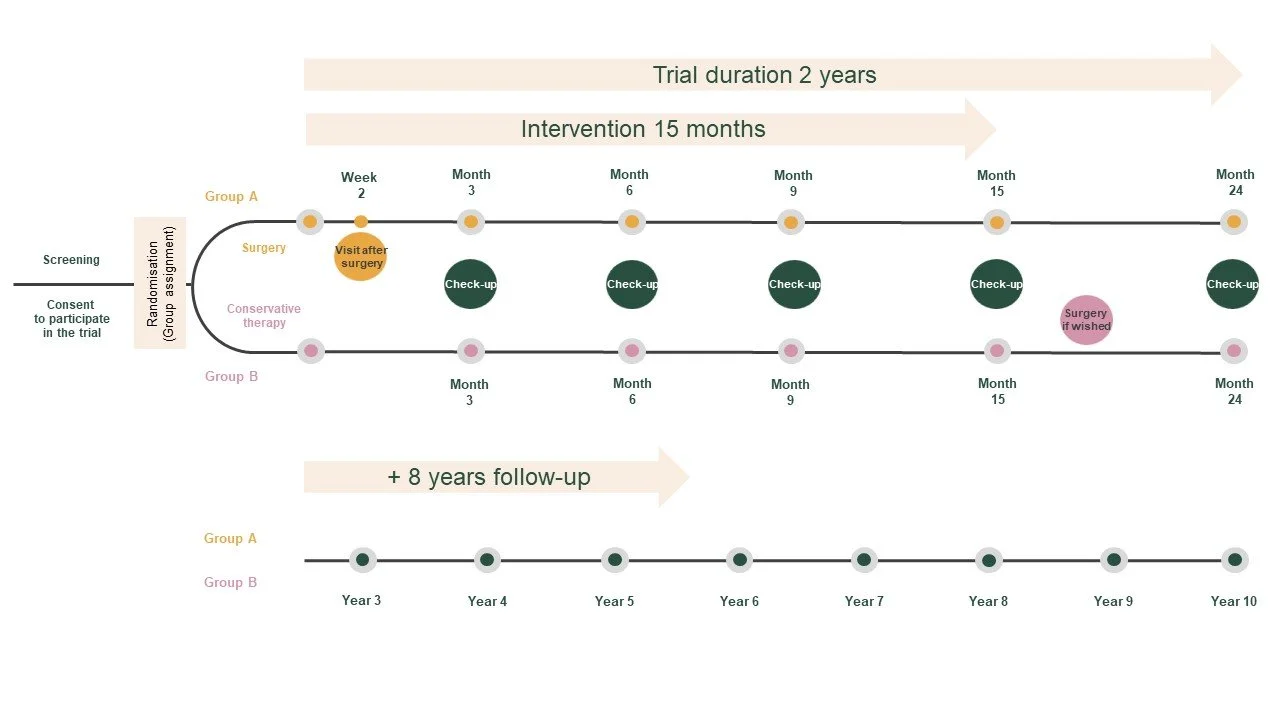
The intervention, whether a) surgery and conservative therapy or b) conservative therapy only, spans 15 months. Regular check-ups occur throughout this period. The trial lasts over a total of 10 years, with regular follow-up examinations during the initial 2 years.
LYMPH-Trial procedure
What is unique about the LYMPH-Trial?
1.
We observe the patients and their quality of life for a long period of time, specifically 10 years! This timeframe surpasses the usual observation period after surgery. This allows us to assess whether patients’ conditions improve over time and whether the therapies we implement are effective in the long term.
2.
Patient representatives played a direct role in setting up the study. Moreover, individuals with chronic lymphedema following breast cancer treatment actively participated in defining the study objectives, ensuring their needs and preferences were considered.
3.
The study team is committed to sharing the results openly with everyone. This means patients and caregivers worldwide can benefit from the findings.
Because together we can make the best choices.
I would like to take part in the study, how do I proceed?
I want to know more about the study!
Frequently asked questions
-
Microsurgical treatment for lymphedema is a common practice in Switzerland and globally. Yet, there is a lack of strong scientific evidence confirming its effectiveness.
Like any surgery, potential side effects of lymphedema surgery may include pain, wound infections, bleeding, haematoma, and swelling.
-
It is important that the study participants are divided into two groups using an “automated lottery procedure”. This is the only way to obtain reliable results. This process is called “randomisation”, in which even the study coordinators do not know in advance which group the participants will be assigned to. Randomisation allows us to assess the results objectively.
-
In principle, our hope is that the study will demonstrate the superiority of surgery over conservative therapy, although conclusive evidence is currently lacking. However, existing studies suggest that maintaining conservative treatment should prevent a worsening of your lymphedema for up to 15 months.
If, during our check-ups, we discover a deterioration in your lymphedema, we will discuss with you the option of undergoing surgery before the 15-month timeframe.
Otherwise, surgery is scheduled upon reaching the “primary endpoint,” which is the attainment of the main study objective —provided it aligns with your preferences.
-
Your participation in the trial will last a total of 10 years, with the actual intervention and follow-up examinations taking place within the first 2 years.
Over these 2 years, you will be invited for approximately 6-9 hospital appointments, depending on your assigned group:
For Group A, consisting of patients undergoing surgery, there are a total of 9 appointments in the first 2 years: 1) Preliminary examination before inclusion in the study, 2) Check-up before the operation, 3) Operation, 4) Check-up 2 weeks after the operation, and 5-9) Follow-up appointments after 3, 6, 9, 15, and 24 months.
For Group B, comprising patients without surgery, there are a total of 6 appointments in the first 2 years: 1) Preliminary examination before inclusion in the study, and 2-6) subsequent check-ups after 3, 6, 9, 15, and 24 months.
Following these initial 2 years, you will be invited to an annual consultation for a period of 8 years for a follow-up check. All 8 of these appointments are integral to your overall treatment and will occur irrespective of your participation in the study.
-
Participating in the trial may enhance the consistency and success of conservative therapy, potentially resulting in an improvement in your lymphedema. By regularly monitoring your quality of life and consistently measuring the lymphedema in a standardized manner may enable quicker responses to any deterioration.
If you are already adhering to a steadfast conservative treatment plan, direct benefits from study participation may be limited.
Timely surgery may address the underlying causes of your lymphedema, offering the potential for improvement.
-
The information about the LYMPH-Trial that you find on this website does not replace the legally required patient information. If you are interested in participating in the study, the study team will provide you with the necessary patient information. The study team will inform you comprehensively about the study, explaining what it means for you to participate.
Detailed information about the LYMPH-Trial in “specialized language” is listed in the study registers ClinicalTrials.gov and Swiss National Clinical Trials Portal (see “For healthcare professionals”).
-
The LYMPH trial is being conducted in various hospitals around the world. You can find an overview under ‘Contact & Sites’
-
You can terminate your participation at any time. You are not required to explain why. Your medical treatment will continue even if you decide to withdraw from the trial. The data collected up to that point will be stored and analysed as part of the trial.
-
We safeguard your data, such as information about your breast cancer diagnosis and treatment from your medical history. Switzerland has strict legal regulations in place to protect this data.
All data is documented in encrypted form to ensure the highest level of protection.